Producing Prevention: How Vaccines Are Developed
As the world struggles to combat and contain COVID-19, the pandemic has underscored the importance of vaccinations.
Recent outbreaks of COVID-19, measles, Ebola and Zika have demonstrated that people are more vulnerable to diseases if they have not been vaccinated against them. For instance, unvaccinated children who are exposed to measles may not be able to resist the disease because their bodies have not had a chance to build antibodies to fight it.
Vaccines help mount a collective public health response to protect the health of communities and countries.
“It’s really a miracle,” Ron Waldman, MD, MPH, a global health professor in the George Washington University’s Milken Institute School of Public Health, said of the development of COVID vaccines. “Everyone who can, should be vaccinated. It’s the way to stop the pandemic.”
Although there is mistrust and misinformation surrounding vaccines, there are rigorous processes that vaccines must go through before they can be proven safe and effective and, ultimately, distributed to the public.
How Vaccines Work in the Body
Though it’s common to hear the terms “immunization” and “vaccine” used interchangeably, they actually describe different things.
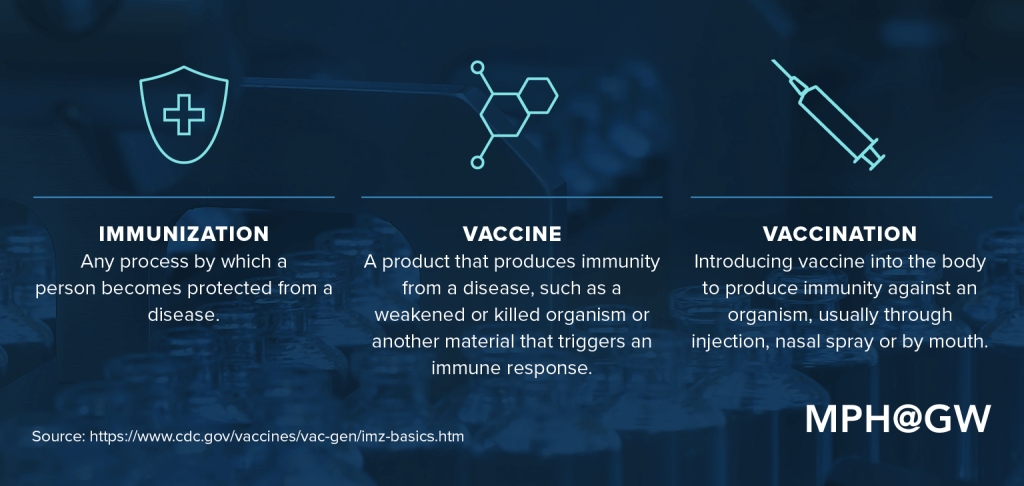
When we get sick, we produce antibodies, or proteins, that help our bodies fight illness. Vaccines weaponize our immune systems by imitating the infection, which in turn prompts our bodies to create the same antibodies it would need to fight a full-blown version of that illness.
It may seem counterintuitive, but the key ingredient in an effective vaccine (PDF, 219 KB) is often an inactive or weakened version of the very bacterial or viral infection it was created to prevent.
“The idea is to take either a related — and less lethal — pathogen, or a weakened version of the disease-causing pathogen itself, and expose the body to that so that it prompts the ability of the body to remember the next time it sees the actual virus or bacteria that you want to protect against, and the body can respond to it right away with a response,” Waldman said.
Some newer vaccines, such as the COVID vaccines developed by Moderna and Pfizer-BioNTech, rely on genetic material to teach the body to recognize and ward off infections, instead of using a weakened virus. These COVID vaccines use messenger RNA (mRNA) — or genetic material that gives instructions — to teach cells to make a spike protein found on the surface of the coronavirus. This allows the body to recognize the infection and fight it off if it encounters the same material in the future, without injecting any actual coronavirus into vaccine recipients.
“The Moderna and Pfizer vaccines are a completely different method of making vaccines from anything that’s been done before,” Waldman said. “The work did not start with COVID. It began many years ago, but the urgency of having something for COVID certainly accelerated the work.”
Contrary to what anti-vaccination communities assert, vaccines are both safe and effective, and mRNA vaccines do not alter a recipient’s DNA. According to the World Health Organization (WHO), severe side effects from vaccines are rare, and individuals are “far more likely to be seriously injured by a vaccine-preventable disease than by a vaccine.”
Vaccine Myth Busting
Although misinformation around vaccines is rampant, the CDC suggests turning to organizations including the National Network for Immunization Information, the Immunization Action Coalition and the Medical Library Association for credible information about vaccines.
“There’s a huge anti-vax movement,” Waldman said. “It’s grown quite a lot during COVID, and there are also organizations that have formed to combat the anti-vax movement.”
Vaccine Myths
COVID Vaccine Myths Versus Facts
Myth: mRNA COVID vaccines will give you COVID-19.
Fact: mRNA COVID vaccines cannot give you COVID-19 because there is none of the COVID virus in them, though side effects of the vaccine may mimic its symptoms.
Myth: Vaccine developers cut corners to create COVID vaccines quickly, so they may not be safe.
Fact: The safety of COVID vaccines was tested in trials on tens of thousands of people before the vaccines were publicly available.
Myth: Receiving an mRNA vaccine will alter your DNA.
Fact: mRNA vaccines deliver genetic material that instructs cells to create an immune response to a disease, but they do not interact with or alter DNA.
Myth: COVID vaccines can cause false positive COVID test results.
Fact: COVID vaccines cannot cause false positive viral (or antigen) tests, though they may cause positive antibody tests if your body develops an immune response to the vaccine.
Myth: You do not need the vaccine if you have already had COVID.
Fact: As of summer 2021, it is unknown how long natural immunity from contracting COVID-19 lasts, but COVID vaccines are predictably safe and effective even for people who have already been sick.
General Vaccine Myths Versus Facts
Myth: Vaccines can cause conditions including autism, cancer and allergies.
Fact: Vaccines are not connected to the development of autism, cancer, allergies or other conditions.
Myth: There is no reason to get immunized against a disease if it is eradicated in your country.
Fact: While certain diseases may no longer be prevalent in your country, some, like measles, still exist worldwide and can reappear if unvaccinated individuals are exposed for any reason, such as coming in contact with travelers.
Myth: Proper hygiene offers better protection against diseases than vaccination.
Fact: While handwashing and other hygiene measures help protect against diseases, vaccination provides a high level of protection and is strongly encouraged by medical experts.
Myth: Getting infected by a disease provides better immunity than vaccination.
Fact: Individuals can gain natural immunity after contracting a disease, but the risk of health problems from contracting a disease is almost always higher than any potential side effects associated with vaccination.
Myth: Vaccines contain unwanted additives, such as microchips that allow governments and big data companies to track individuals, toxic substances like mercury or birth control agents to control the populations of developing countries.
Fact: Vaccines do not contain toxic chemicals, microchips or birth control. Some vaccines are manufactured with low levels of the mercury-based preservative thimerosal, which has been shown to be safe in vaccines, according to the CDC.
Sources:
- “COVID-19 Vaccine Myths vs. Facts,” GW School of Medicine & Health Sciences. June 3, 2021. Accessed June 28, 2021.
- “Myths and Facts about COVID-19 Vaccines,” Centers for Disease Control and Prevention. June 23, 2021. Accessed June 28, 2021.
- “COVID-19 Vaccine Myths,” (PDF, 189KB), American Academy of Family Physicians. Accessed June 28, 2021.
- “Myths and facts about immunization,” (PDF, 150KB), World Health Organization Regional Office for Europe. Accessed June 28, 2021.
The Path to Prevention
The effectiveness and life-saving nature of vaccines leads people to pose the same question whenever a public health crisis, such as COVID-19, Zika or Ebola, hits the news cycle: “When will a vaccine be available?”
The answer, which also considers which types of professionals are needed at each stage of development, is complicated. There are six stages of vaccine development, according to the CDC: exploratory, pre-clinical, clinical development, regulatory review and approval, manufacturing and quality control.
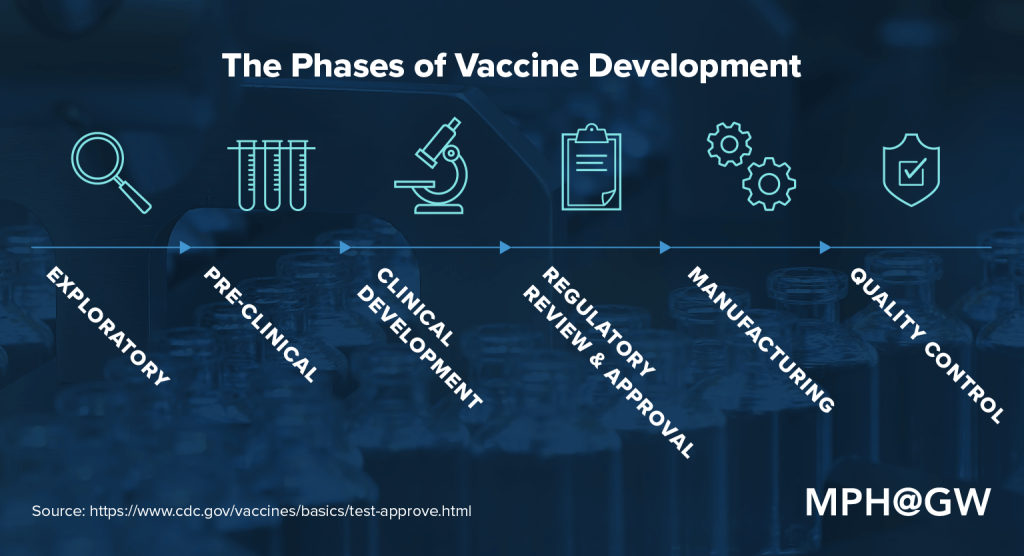
Exploratory: This research-intensive phase of the vaccine development process is designed to identify “natural or synthetic antigens that might help prevent or treat a disease.” Antigens might include weakened strains of a particular virus.
Pre-clinical: During this phase, researchers — usually in private industry — use tissue-culture or cell-culture systems and animal testing to determine whether the candidate vaccine will produce immunity. Many candidate vaccines don’t move on to the next stage of development because they fail to produce that immunity or prove harmful to test subjects.
Clinical development: At this point, a sponsor, usually a private company, submits an application for an Investigational New Drug (IND) to the U.S. Food and Drug Administration (FDA). This summarizes findings to date and describes how the drug will be tested and created. An institution that will host the clinical trial holds a review board for approval of the application. The FDA has 30 days to approve the application. Once the proposal has been approved, the vaccine must pass three trial stages of human testing:
- Phase I administers the candidate vaccine to a small group (fewer than 100 people) with the goal of determining whether the candidate vaccine is safe and to learn more about the responses it provokes among test subjects.
- Phase II, which includes hundreds of human test subjects, aims to deliver more information about safety, immunogenicity, immunization schedule and dose size.
- Phase III, which can include thousands or tens of thousands of test subjects, continues to measure the safety (rare side effects sometimes don’t appear in smaller groups) and effectiveness of the candidate vaccine.
Regulatory review and approval: If a vaccine passes through all three phases of clinical development, the vaccine developer submits a Biologics License Application (BLA) to the FDA. In emergency scenarios, the FDA can grant Emergency Use Authorization (EUA) for the use of unauthorized medical products. The COVID vaccines in use in the United States were given EUA.
Manufacturing: Major drug manufacturers provide the infrastructure, personnel and equipment necessary to create mass quantities of vaccines. They also reap the profits of successful or widely distributed drugs.
Quality control: The approval and distribution is far from the end of the line. Stakeholders must adhere to procedures that allow them to track whether a vaccine is performing as anticipated. Multiple systems — including Phase IV trials (optional studies that can be conducted following the release of a vaccine), the Vaccine Adverse Event Reporting System (VAERS) and the Vaccine Safety Datalink — are designed to monitor the performance, safety and effectiveness of an approved vaccine.
Skeptics have questioned the safety of COVID vaccines because they were developed so quickly and have not yet been licensed by the FDA. The process to develop past vaccines has typically taken years.
“So, why is this an exception? Well, for one thing, we need it really badly. There’s an urgency to it,” Waldman said. “What has to happen is that scientists have to look at the data that’s been generated from the rapidly assembled and implemented trials very, very carefully and see if the data regarding safety and effectiveness holds up as we move forward vaccinating people in real life.”
Who Is Involved in Vaccine Production?
Vaccine development requires careful work from trained professionals in a variety of sectors throughout the process. Certain people can wear many hats. An academic researcher, for instance, may also hold a position at a nongovernmental organization (NGO) and influence the way in which that institution works with manufacturers. As another example, a pharmaceutical company might employ a health professional who previously worked for a federal agency. With that in mind, some of the major stakeholders — people and organizations who directly affect the production process — include health professionals, manufacturers, NGOs, academia, government agencies, media, and individuals and communities.
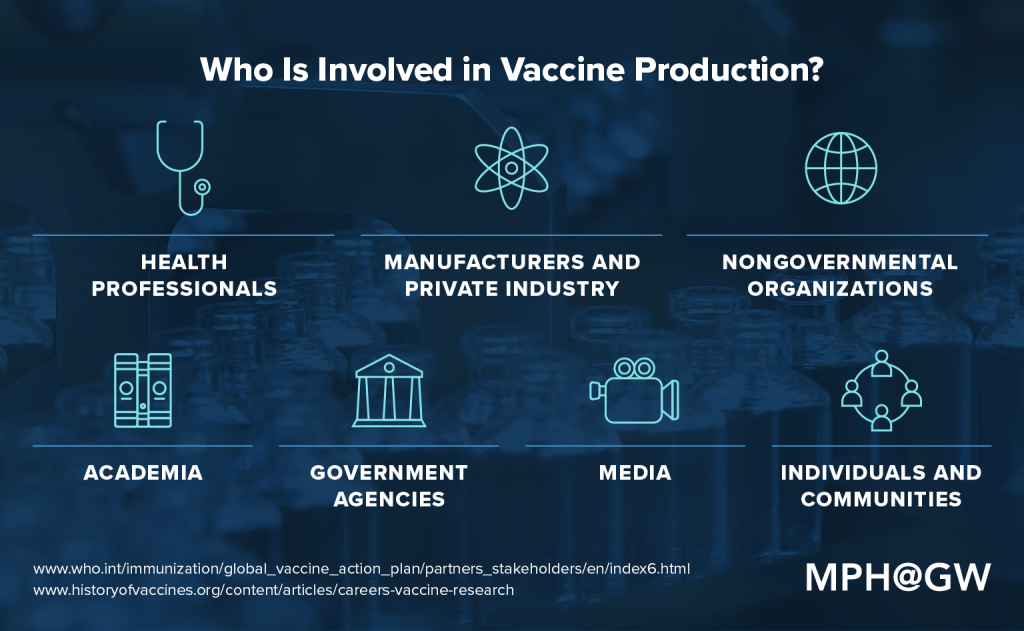
Health professionals: Those with advanced degrees in biology, chemistry, epidemiology, medicine and a variety of other health fields play a crucial role in identifying and vetting vaccines. They also serve as subject matter experts throughout the approval and regulation process. According to The History of Vaccines, those leading the research in vaccine development typically have “a doctorate degree in cellular and molecular biology, biochemistry, or microbiology”; however, these professionals also require help with an array of research functions that can be conducted by people who have bachelor’s or master’s degrees in those subject areas.
Manufacturers and private industry: Developing and testing a vaccine are expensive processes and rife with the potential for liability, which means that funding must come from private institutions, such as pharmaceutical companies.
Nongovernmental organizations (NGOs): These institutions can act as an important conduit between communities and manufacturers with the goal of easing communication in regions where a vaccine is needed. This might include working directly with populations to facilitate immunization in an equitable way that accounts for social determinants, collaborating with local health systems and health workers, and funding the provision of vaccines.
Academia: The academic community conducts valuable research that has the potential to help other stakeholders understand what can be learned about myriad aspects of the vaccination development process. They may be able to advocate for evidence-based immunization practices, reflect on the socioeconomic implications of a vaccine for an at-risk community or synthesize past research.
Government agencies: In the United States, federal agencies such as the FDA must serve as the check and balance for any vaccine that will be made available to the general public.
Media: Though members of the media may not be “hands-on” stakeholders, they can elevate the profile of a certain disease or condition that requires a vaccine. Reporting the details of a vaccine’s progress, challenges or dangers provides an important public service. At the other end of the spectrum, however, the media can exacerbate unfounded fears around certain diseases or catalyze unrealistic expectations for the timing of a vaccine’s availability.
Individuals and communities: The general public plays a crucial role in determining the safety and effectiveness of a vaccine. From participating in trials to serving as end users for an approved drug, understanding the real-world implications of a vaccine comes down to this group.
Given the meticulous testing and approval protocols involved in vaccine development — as well as the sheer number of stakeholders involved — it can take years to move a vaccine from a lab to the general public. That time line, however, can change when a public health crisis, such as an outbreak or a natural disaster, emerges suddenly.
During a pandemic such as COVID, getting vaccines to parts of the world that need them most but do not have access to them presents another challenge.
“The WHO has said this represents potentially the greatest moral failure that the world has ever experienced — that you protect some (those in wealthier nations) and not others,” Waldman said. “We have this tremendously inequitable distribution of this miraculous tool that, if used appropriately, could bring this pandemic to an end.”
Considering a Career in Public Health?
Through our commitment to excellence in scholarship, we advance the health of populations in our local, national and global communities by providing the best public health educational experience to foster the next generation of public health leaders.
Citation for this content: MPH@GW, the online MPH program from the Milken Institute School of Public Health at the George Washington University.


